Create Date: June 29, 2024
Last Modified Date: January 28, 2025
The way you calculate the boiling point of water at a specific altitude is with this formula:
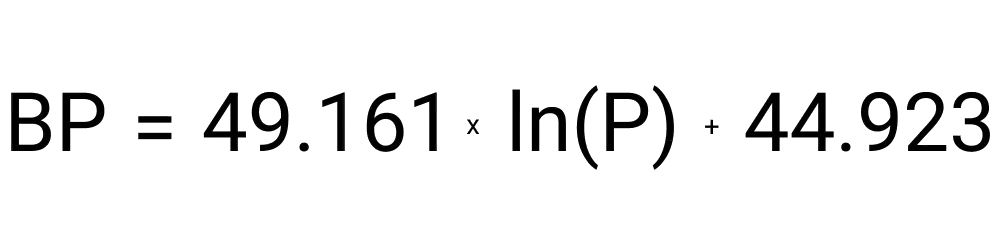
Our tools are designed to be as simple and easy to use as possible. Our altitude boiling point tool is just that. The steps involved with using this tool includes:
The boiling point of water will be affected due to the air pressure at your elevation. With a higher elevation comes less air pressure, this means it takes less energy to get water to reach the boiling point and vice versa.
When the boiling point of water goes down, it also affects the way food cooks. You will need to cook your food longer than you normally would, so adjusting your recipes for your altitude is crucial to make sure your food is thoroughly cooked and safe to eat.
Let's say we are going to Denver and want to see what the boiling point temperature would be with the higher elevation. We can find that with this tool. We are going to be staying at a place that is 5,000 feet above sea level so we can enter 5,000 into the altitude field.
We can leave the unit as feet since that is accurate and then hit calculate. We get an answer of 202.37 degrees Fahrenheit, or 94.65 degrees Celsius.
The connection between altitude and how it affects the boiling point of liquids was not something that was found in the early days. Despite Aristotle being very smart and advanced for his time, any connection between altitude and boiling point would not be discovered or even looked into until around the 1600s.
In the year 1643 the barometer was invented by Italian physicist Torricelli which would become the groundwork for making the connection between altitude and boiling points. Later in the 1660s, Robert Boyle studied the relationship between pressure and has behavior, indirectly linking the concepts of altitude and boiling points.
We inched closer to coming to this conclusion when in the 1680s Denis Papin invented the steam digester, which is similar to a pressure cooker because he noticed that pressure influenced the boiling point of water. The most clear connection was made by Alexander von Humboldt in the late 1700s. He was on a scientific expedition and recorded the boiling point variations at different altitudes.
In 1802 Joseph Louis Gay-Lussac would expand on previous recordings by directly proving that boiling points decrease at higher altitudes. This is now a well known fact and is widely reported and studied.
Yes, at higher altitudes, where the boiling point of water is lower, it can take longer to cook food thoroughly. This is because water boils at a lower temperature, providing less heat for cooking.
When cooking at high altitudes, you may need to increase cooking times and temperatures. For baking, adjustments might include increasing the amount of flour or decreasing the amount of baking powder and sugar to accommodate for faster rising due to lower air pressure.
The lower boiling point can affect the texture and flavor of food. Foods may cook differently, often requiring longer cooking times which can alter their expected taste and texture.
Understanding boiling point at altitudes can be difficult if some of the terms and keywords used are not ones you understand. Here we shed some more light on some of these terms.
| Term | Definition |
|---|---|
| Altitude | Altitude refers to the height an object or subject is from sea or ground level. |
| Barometer | A tool that was invented to measure pressure which uses mercury to determine if there is any pressure being exerted at a given time. |
| Elevation | Synonymous with altitude, elevation is the height something is over a certain point, typically sea or ground level. |
There are many interesting things that can be shared about boiling points. Here are some of our favorites.
At the top of Mount Everest (8,849 meters or 29,032 feet), water boils at about 154°F (68°C). At ground level it boils at about 212°F.
In the vacuum of space, water doesn’t "boil". It transitions directly from liquid to gas in a process called sublimation, making it have no boiling point.
In the 19th century, mountaineers and explorers used boiling water thermometers to measure altitude. The lower the boiling point, the higher they were.