Create Date: July 1, 2024
Last Modified Date: January 14, 2025
Molarity can be calculated with the following variables:
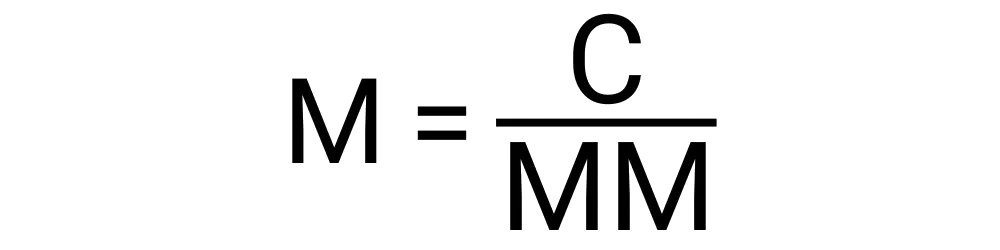
Molarity is used extensively to prepare solutions for laboratory experiments and industrial processes. It ensures that chemical reactions occur under controlled and repeatable conditions.
For example, when a chemist needs to carry out a reaction that requires an exact amount of reactant, knowing the molarity of the solutions involved allows precise measurement of volumes instead of weights, simplifying the process and increasing accuracy.
Calculating molarity has never been easier. With this tool you can easily find molarity and for free as well. The steps involved with using this tool include:
Understanding molarity through examples can help clarify its application:
To calculate molarity, you divide the number of moles of the solute by the volume of the solution in liters. The formula is: Molarity (M) = Moles of solute / Volume of solution in liters.
To prepare a solution with a specific molarity, first calculate the number of moles of solute needed using the desired molarity and the volume of the solution. Then, measure out that amount of solute, usually in grams, and dissolve it in a portion of the solvent. Once the solute is fully dissolved, adjust the volume of the solution to the exact final volume with additional solvent.
Molarity is the concentration of a solution expressed as moles of solute per liter of solution. In contrast, molality is defined as the moles of solute per kilogram of solvent. Molality is not affected by temperature changes, unlike molarity, because it depends on the mass of the solvent rather than its volume.
In a laboratory, molarity can be measured using a volumetric flask for solution preparation, a balance for weighing the solute, and a spectrophotometer or titrator for assessing the concentration of the solution post-preparation if necessary.