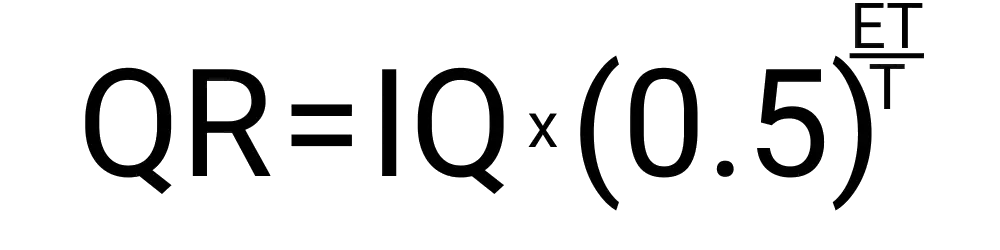The History of Half-Life
The concept of half-life is not one that goes back thousands of years. Some of the earliest roots for half-life can be traced back to 1896 when Henri Becquerel discovered that uranium salts emitted radiation without external energy input, making it the first observation of radioactive decay.
Just a couple of years later, in 1898, Marie and Pierre Curie made the discovery of polonium and radium which would show that some elements naturally break down into other elements over time. This would begin the conversation around a new concept where elements were not eternal but could transform into other elements.
The true first steps to understanding half-life and naming its existence happened around 1900. Physicist Ernest Rutherford and chemist Frederick Soffy discovered that radioactive elements decayed into different elements at fixed rates. They would introduce the idea of an exponential decay process. It wouldn't be until 1907 when the term half-life would be created by Rutherford.
The concept has had extensive research and experimentation since then, over a century of different scientists and minds coming together to learn more about the topic. The concept also would be used in many other areas of study and experimentation and is a major piece of information today.
Half-Life - Frequently Asked Questions
A half-life of 2 hours means it will take 2 hours for a substance to reduce its quantity to half of its current amount.
No, half-life cannot be negative as it represents a positive time interval.
Tellurium-128 has an exceptionally long half-life of 2.2 x 1024 years, far exceeding the age of the universe.
Page Glossary
Understanding half-life can be difficult if some of the terms and keywords used are not ones you understand. Here we shed some more light on some of these terms.
| Term |
Definition |
| Half-life time |
This is the amount of time that it takes for a radioactive element to decay by half. |
Similar Tools
Related Calculators
We have many more calculators that you can use for free. Here are a few similar tools that might interest you.