Neutralization Calculator
Neutralization in Chemistry refers to a chemical reaction where an acid and a base interact with each other. Use this calculator to help you find the normality value in a situation like this.
Required Information
Result:
Create Date: September 5, 2024
Last Modified Date: January 14, 2025
Neutralization is calculated with the following variables:
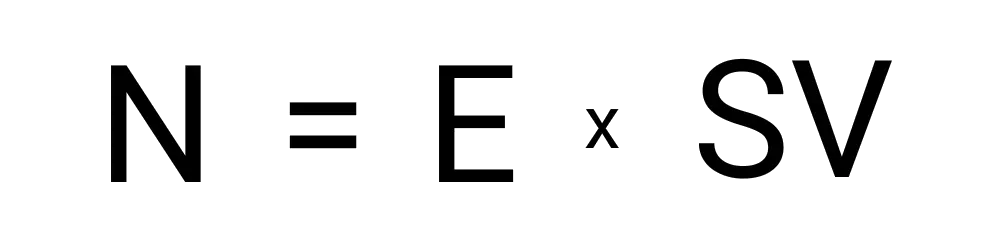
The steps involved with using this include:
The way to tell if a reaction is a neutralization reaction is if water and salt are products of it.
Depending on the amount of acid, sodium bicarbonate (baking soda) can be used to neutralize sulfuric acid effectively.