Water Heating Calculator
We do not often think of the amount of energy that was required to heat the water that we use. Use this tool to find out just how much energy was required to heat said water.
Required Information
Total Energy:
Create Date: October 3, 2024
Last Modified Date: January 15, 2025
When water gets heated there are a number of variables that we would need to utilize to discover how much energy it took to heat the water up. The variables include:
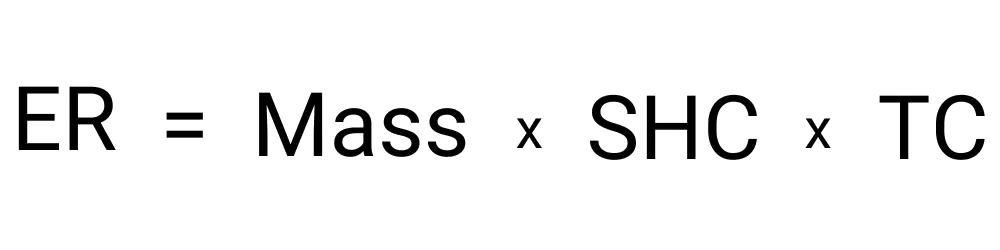
The result you get will be the total amount of energy required to heat the amount of water you entered by the amount of degrees that you entered. This value is displayed as a single number and is shown in joules by default but can be converted easily into other units of energy measurement, if needed.
Our goal is to make the best and most efficient tools for you to use. We make our tools simple and easy to use so you get your answer as fast as possible. The steps involved with using this tool include:
Let's say you have a total of 50 ounces of water. You observed its temperature rise from 50 degrees Fahrenheit to 60 degrees Fahrenheit. To find out how much energy it took for this change in temperature to happen we can use this tool. We enter 50 into the amount of water field, 50 in the initial temperature field and 60 in the final temperature field. We do not have to change any of the units of measurement so we are ready to hit calculate.
When we hit calculate we find out that the total energy required for that water heating is 32,964.17 joules. We can easily convert this to another unit by selecting it from the dropdown list, if needed.
The specific heat capacity of water is approximately 4.18 joules per gram per degree Celsius (J/g °C). It means that it takes 4.18 joules of energy to heat one gram of water by one degree Celsius.
Yes, boiling water requires significantly more energy compared to just heating it, because boiling involves changing the state of water from liquid to gas, which requires overcoming intermolecular forces.
Heating a larger amount of water takes longer because there are more water molecules that need to absorb energy to increase in temperature. More energy is needed to heat larger volumes of water compared to smaller volumes.